Alimetry Secures CPT III Reimbursement Code for the Gastric Alimetry Test and Announces Positive Clinical Study Results
Alimetry, a gastrointestinal medtech and digital health start-up, announced today the creation of a new American Medical Association (AMA) Current Procedural Terminology (CPT) III code for Body Surface Gastric Mapping, which will describe its Gastric Alimetry test.
The new CPT III code highlights the growing recognition of Body Surface Gastric mapping and Gastric Alimetry as a significant advance in the diagnosis and management of chronic gastric conditions, including functional dyspepsia, gastroparesis, and chronic nausea and vomiting syndrome. Together, these disorders affect >10% of the US population.
CEO, Greg O’Grady, a Professor of Surgery and co-founder of Alimetry, said: “Obtaining a CPT III code is a key milestone for Alimetry. Gastric Alimetry is emerging as a next-generation test for patients suffering chronic gastric symptoms, with excellent early adoption in over 30 hospitals and clinics. The new AMA code will accelerate our mission to achieve widespread access for US patients suffering from these disabling disorders.”
The CPT III code comes on the back of Alimetry’s 3rd 510k clearance from the US Food and Drug Administration (FDA) in just 2 years for the Gastric Alimetry System. The latest FDA clearance introduced new features to aid in the diagnosis of gut hypersensitivity and common ‘Gut-Brain-Axis’ disorders, which are often linked to mental wellbeing. Chief Medical Officer Professor Chris Andrews stated: “Gastric symptoms can be complex, and are often a diagnostic challenge. Gastric Aimetry combines a wearable sensor with an App- based digital health profiling tool, providing an increasingly powerful feature set that can greatly simplify the diagnostic process. The cloud-based platform also enables us to rapidly learn and improve test performance based on banked data, with an increasing focus on applications in AI.”
In addition to the new CPT Code, Alimetry also announced positive clinical study results, published this month in the American Journal of Gastroenterology. In the study discussion, the authors concluded that “Gastric Alimetry identified 2.7x more specific patient categories than gastric emptying testing [a standard of care test], with limited overlap between each diagnostic modality, offering a valuable new option in the diagnostic work up of patients with chronic gastroduodenal symptoms”. In addition, Gastric Alimetry data was reported to show “improved correlation with symptoms and psychometrics” compared to existing clinical and research tools.
Gastric Alimetry is commercially available in the United States, the UK and New Zealand. The study article can be accessed in this hyperlink
About Gastric Alimetry
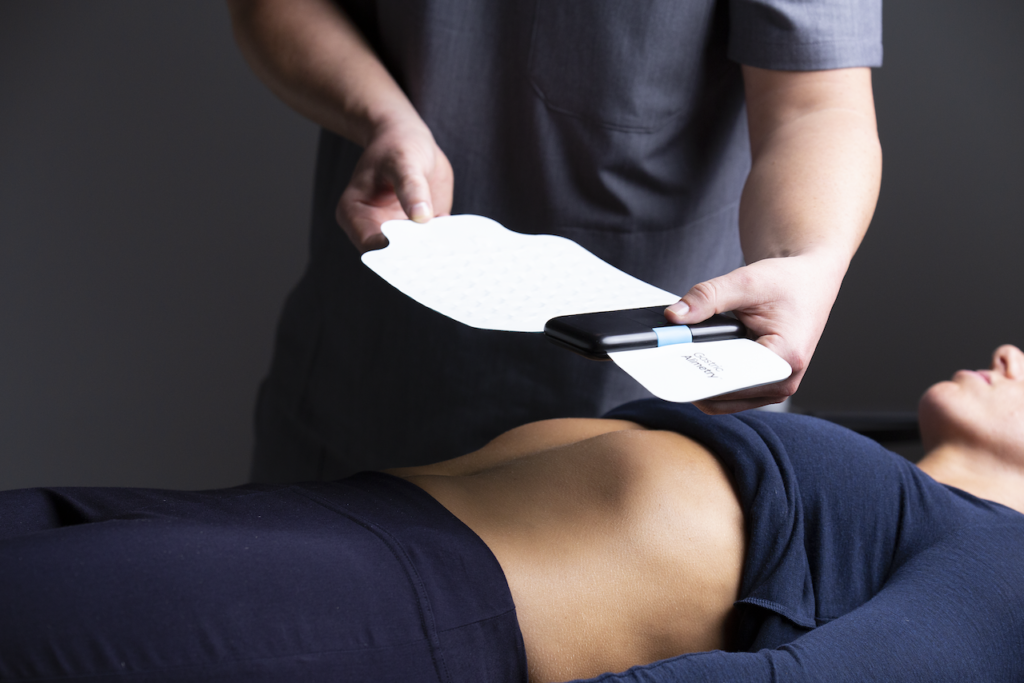
The Gastric Alimetry test measures gastric electrophysiology non-invasively in a clinical setting. Recordings are taken before and after a standard meal, while patients simultaneously log their symptoms into the Gastric Alimetry App. The system performs a high-resolution recording of digestive patterns from the skin surface and delivers clinical reports via the cloud to inform the diagnosis of gastric diseases and support personalized therapy. The Gastric Alimetry system is indicated as an aid to diagnosis of various gastric disorders, including functional dyspepsia, gastroparesis and chronic nausea and vomiting disorders, which affect >10% of the US population and impact billions of dollars in healthcare expenses.
About AMA CPT III Codes
AMA CPT III codes are temporary codes assigned to emerging medical technologies and procedures. These codes facilitate data collection and assessment, aiding in the validation and reimbursement of new services. Obtaining a CPT III code, such as for Alimetry’s Gastric Alimetry test, is a vital step towards recognizing its clinical relevance and supporting its integration into healthcare practices. The new CPT III code number is 0868T and it is effective from July 1, 2024.
About Alimetry
Alimetry is a medtech and digital health start-up focused on improving the care of patients with chronic gastrointestinal symptoms. Spun out of the University of Auckland in 2019 from a centre of excellence in digestive diseases, the company was founded by Professor Gregory O’Grady (CEO) and Dr. Armen Gharibans (CTO), leveraging a decade of award-winning science. Alimetry is dedicated to advancing gastrointestinal health through innovative diagnostic solutions, aiming to enhance the quality of life for patients worldwide. For more information see www.alimetry.com
Contact
Hanie Yee, Chief Commercial Officer, Alimetry Hanie@alimetry.com
NZ +64 (0)21 651226 US +1 (657) 267 1082
Alimetry announces US FDA Clearance for Gastric Alimetry – A Breakthrough Non-Invasive Wearable Device for Gut Diagnostics
Auckland, New Zealand – Alimetry, a medical device and digital healthcare company, today announced it has received US Food and Drug Administration (FDA) clearance for Gastric Alimetry, a pioneering non-invasive medical device for aiding the diagnosis gastric disorders. Alimetry also announced today the launch of Alimetry Inc., a subsidiary based in Minneapolis, MN, that will market and distribute Gastric Alimetry in the United States.
“Gastric Alimetry is an industry-first and genuine breakthrough in gut diagnostics.” said CEO Greg O’Grady, a Professor of Surgery and co-founder of Alimetry. “Alimetry’s unique technology harnesses the power of stretchable electronics, wearables, digital health, and cloud-based analytics to deliver a completely non- invasive solution. We are thrilled to announce FDA’s clearance of Gastric Alimetry, making this new test available to millions of Americans suffering from chronic gastric symptoms”.
The Gastric Alimetry test is performed in a clinical setting. Recordings are taken before and after a meal, while patients simultaneously log their symptoms into the Gastric Alimetry App. The system performs a high-resolution recording of digestive patterns from the skin surface and delivers clinical reports via the cloud to inform the diagnosis of gastric diseases and support personalized therapy.
The system is indicated for common stomach disorders including nausea and vomiting, gastroparesis, and functional dyspepsia, affecting over 8% of the world’s population, and costing billions of dollars in healthcare expenses.1,2 The test is also currently available in the United Kingdom and New Zealand.
“Diagnosing gastric symptoms has been a deeply challenging clinical problem.” Dr. O’Grady said. “Existing tests are frequently unreliable and inconclusive, and patients may undergo months or even years of testing – often costly, invasive, or involving radiation – only to end in confusion and trial-and-error care. Gastric Alimetry is a game-changing tool that will bring improved clarity to field, enabling enhanced clinical outcomes, and safer, more accessible, and less-invasive care.”
Gastric Alimetry is the result of a decade of world-leading science and innovation by an interdisciplinary team of clinicians, engineers, designers and scientists.
“FDA clearance of Gastric Alimetry cements Alimetry’s path to leadership in gastrointestinal wearables and non-invasive diagnostics.” Dr. O’Grady said. “Alimetry has an outstanding pipeline of innovation and this is the first plank in a series of innovative new features and products that stand to transform care in disorders of gastrointestinal function”.
Gastric Alimetry will be available commercially from July 2022.
For more information on Gastric Alimetry visit www.alimetry.com
About Alimetry
Alimetry was spun-out of the University of Auckland in 2019 from a centre of excellence in digestive diseases. The company was founded by Professor Gregory O’Grady, a gastrointestinal surgeon, and Dr. Armen Gharibans, on the background of a decade of award-winning science.

References
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology. 2021 Jan 1;160(1):99-114.
- Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Alimentary pharmacology & therapeutics. 2013 Jul;38(2):170-7.
Alimetry raises $16.3 Million to launch wearable diagnostic device for the gut
Alimetry, a digital healthcare and medical device start-up, announced today it has closed a NZ$16.3 Million financing round led by New Zealand largest venture capital investor Movac. Existing investor partners IP Group, Matū Karihi, and UniServices supported the round, with K1W1 also participating.
The company will use the funding to accelerate the international launch of its first product, a wearable diagnostic device called Gastric Alimetry. Gastric Alimetry uses a stretchable high-resolution sensor to non-invasively map digestive patterns, and delivers clinical reports via the cloud to inform the diagnosis and classification of gastric disorders.
“Chronic gastric symptoms are extremely common, yet many of our patients still suffer from repeated inconclusive tests, trial and error care, and confusion,” said CEO Greg O’Grady, a Professor of Surgery and co-founder of Alimetry. “Gastric Alimetry is on track to deliver breakthrough results in diagnosing gastric symptoms, enabling enhanced clinical outcomes and safer, more accessible, and less-invasive care.”
Gastric Alimetry targets prevalent stomach diseases including nausea and vomiting, gastroparesis, and functional dyspepsia, affecting over 8% of the world’s population and costing billions of dollars in healthcare expenses. The device is currently undergoing clinical trials in 5 countries, with results and further regulatory approvals expected during 2022. Alimetry will also use the new funding to expand its artificial intelligence capabilities and expand its product pipeline.
Lovina McMurchy, a General Partner at Movac who will join Alimetry’s Board, stated: “While Alimetry has only recently come out of stealth mode in New Zealand, the team has made an incredible amount of progress in bringing their product to market globally. They are already working with the top gastroenterology clinics in the world to trial the devices and they are in the approval process with the Federal Drug Administration in the US for full scale commercialization. This is a tremendous example of what our science ecosystem can produce at it’s very best.”
Gastric Alimetry has recently received numerous innovation and product design awards, including Gold from the Australian Good Design Awards, and a prestigious ‘Purple Pin’ at the New Zealand Best Design Awards. The Best Awards judges stated: “This is a tangible example of how New Zealand science, technology and design can work together to produce brilliant results.”
Alimetry was spun-out of the University of Auckland in 2019 from a centre of excellence in digestive diseases. The company was founded by Prof. Greg O’Grady and Dr. Armen Gharibans, on the background of a decade of award-winning science.
To learn more about Alimetry visit www.alimetry.com.
About Movac
Movac is New Zealand’s largest and most experienced venture capital fund, supporting technology founders from early stage through to growth investing. Movac was the first institutional grade venture investor supported by both New Zealand Superannuation and Kiwi Wealth. It currently has $400m funds under management and invests across deep tech, software, hardware and healthtech.
About IP Group
IP Group is a leading intellectual property commercialisation company focused on evolving great ideas and cutting-edge research from its partner universities into world-changing businesses. The Group pioneers a unique approach to developing these ideas and the resulting businesses by providing access to business building expertise, capital, scientific insight, and supporting infrastructure. IP Group, which is listed on the London Stock Exchange (LSE: IPO), has a strong track record of success and, its portfolio comprises holdings in early stage to mature businesses across life sciences and technology. In Australia and New Zealand, IP Group works in close partnership with the Go8 Universities and the University of Auckland to identify ground-breaking technologies rooted in hard science, which have the most promising commercial potential. www.ipgroupanz.com
About University of Auckland Inventors’ Fund, managed by Auckland UniServices
The University of Auckland Inventors Fund is an evergreen, open-ended $20 million investment fund owned and managed by Auckland UniServices Limited, the commercial company for The University of Auckland. The Inventors’ Fund provides seed capital for ventures started out of the University of Auckland. www.uniservices.co.nz
About Matū Karihi Fund.
Matū is a venture capital fund investing in early-stage science and deep technology commercialisation from education and research institutions and the private sector. Karihi (nucleus) is Matū’s original pre-seed and seed-focused fund, working with start-ups at the earliest stages of their journey. As an open fund it raises capital over time and holds investments through to exit where possible, and therefore invests on a long timeframe. www.matu.co.nz
About K1W1
K1W1 Ltd is an investment company owned by Sir Stephen Tindall. It has invested over a total of $100M Seed and Venture capital into a large number of start-up and early stage businesses from Biotech, environmental technology, high tech, software and other high export potential businesses. The aim is, either directly or as a “fund of funds” to assist young entrepreneurs to grow New Zealand as a leader in the “knowledge economy” and to help create a culture of making New Zealand “cash flow positive” in international goods and services trade.
Attached files: Gastric Alimetry-11
Alimetry Leaves Stealth Mode to Announce CE Mark on New Wearable Medical Device for Gastric Diseases and Investment
Alimetry, a digital healthcare and diagnostic devices start-up, announced today that it has achieved CE Mark for its first product, a pioneering medical device for enabling diagnosis of gastric diseases.
The new wearable product, called Gastric Alimetry, is positioned to transform the diagnostic pathway for millions of patients worldwide suffering from diseases such as functional dyspepsia, gastroparesis and chronic nausea and vomiting. Gastric symptoms are extremely prevalent and impart a vast burden, affecting around 10% of the global population. The Gastric Alimetry device collects data by non-invasively sensing the activity of the stomach from the body surface. The data is sent to the cloud for analysis, and is used by clinicians to determine the causes of gastric symptoms and direct treatment.
Co-Founder and CEO Professor Greg O’Grady said “As clinicians, we lack the tools we need to reliably diagnose gastric disorders. This contributes greatly to the frustration and suffering of our patients. I was driven to this cause by seeing too many patients go through laborious, invasive and expensive rounds of repeat diagnostic testing, only to end up with inconclusive results and confusion. We invented Gastric Alimetry to help address this need. I am immensely proud of our hard-working team who have delivered an incredibly creative solution with outstanding potential to impact patient care.”
In addition to successfully achieving CE mark for the Gastric Alimetry product, enabling the company to start commercializing the medical device in European countries, Alimetry also announced today that it had achieved the ISO 13485 international quality management system accreditation. As it grows global operations, the company has enhanced its executive team with new appointments. Hanie Yee joined the company as Chief Operating Officer, from her previous role as Clinical Business Lead at Fisher & Paykel Healthcare, and Professor Chris Andrews, a leading gastroenterologist from Canada, has joined Alimetry as Chief Medical Officer.
Alimetry has been the culmination of over a decade of award-winning scientific research out of The University of Auckland, backed by scientific grants from the New Zealand Health Research Council and the US National Institutes of Health, followed by Callaghan Innovation funding. To support the next phase of growth, the company completed its first institutional investment round, led by IP Group, a leading international intellectual property commercialisation company. The investment round was supported by UniServices Ltd (via the University of Auckland’s Inventors’ Fund), and Matū, a New Zealand early-stage science and deep-tech venture capital fund. The capital raised by Alimetry will be used to advance the company’s clinical trials, enter the market, and progress regulatory approval in the United States.
The Managing Director of IP Group Australia, Dr Michael Molinari, said “We are excited to be working with Professor O’Grady and the world-class team at Alimetry to provide a step change in the quality of life for millions of patients with gastric disorders. This technology, at the intersection of multiple exponentially growing fields such as wearable medical devices, digital health, and machine-learning assisted diagnostics, is another great example of the breakthrough innovations coming from our partners at the University of Auckland”.
About Alimetry
Alimetry was founded in 2019 as a spin-out company from the University of Auckland’s Bioengineering Institute and Faculty of Medical and Health Sciences. The company was founded on a background of world-leading science in gastrointestinal diseases. Alimetry is dedicated to improving the lives of patients by delivering innovative medical solutions to advance GI diagnostics and enable targeted therapies. www.alimetry.com
About IP Group
IP Group is a leading intellectual property commercialisation company focused on evolving great ideas from its partner universities into world-changing businesses. The Group pioneered a unique approach to developing these ideas and the resulting businesses by providing access to business building expertise, capital, scientific insight, and the supporting infrastructure. In Australia and New Zealand, IP Group works in close partnership with the Go8 Universities and the University of Auckland to identify ground-breaking technologies, rooted in hard science, which have the most promising commercial potential. IP Group, which is listed on the Main Market of the London Stock Exchange under the symbol IPO, has a strong track record of success and its portfolio comprises holdings in early stage to mature businesses across life sciences and technology.
Discover more at www.ipgroupanz.com
About The University of Auckland Inventors’ Fund. The University of Auckland Inventors Fund is an evergreen, open-ended $20 million investment fund owned and managed by Auckland UniServices Limited, the commercial company for The University of Auckland. The Inventors’ Fund provides seed-capital for ventures started out of the University of Auckland. www.uniservices.co.nz
About Matū Fund. Matū is a venture capital fund investing in early-stage science and deep technology commercialisation from education and research institutions and the private sector. As an open and evergreen fund, Matū takes a long-term investment view and is aimed at turning ground-breaking ideas into globally focused, IP-rich companies. Matū provides intelligent capital with active governance, executive management, operational support, and mentorship for founding and executive teams. www.matu.co.nz
Released images (png format):
Contact Information:
Hanie Yee, Chief Operating Officer
M: +64 (0) 21 651 226
Social Media:
Twitter: @alimetry_ltd

